Trust Begins With Safety and Transparency

At Longevity Medical Institute®, trust is not a slogan, it is something we earn through federal licensing, measurable science, and physician-led care. In a field where “stem cells” are often marketed without clear standards, patients deserve certainty about three things:
- Is the clinic federally authorized by COFEPRIS (Mexico’s FDA) to do this?
- What is actually being administered?
- Who is making the medical decisions, and what qualifies them to lead?
This month’s newsletter is built around the three pillars that define trust in regenerative medicine:
Pillar 1: Federal licensing and regulatory oversight - and why it matters more than most patients realize.
Pillar 2: Transparency at the cellular level - made possible through our ISO-certified biotechnology laboratory and flow cytometry reporting.
Pillar 3: Physician-led regenerative medicine - treatment decisions guided by highly trained regenerative medicine physicians.
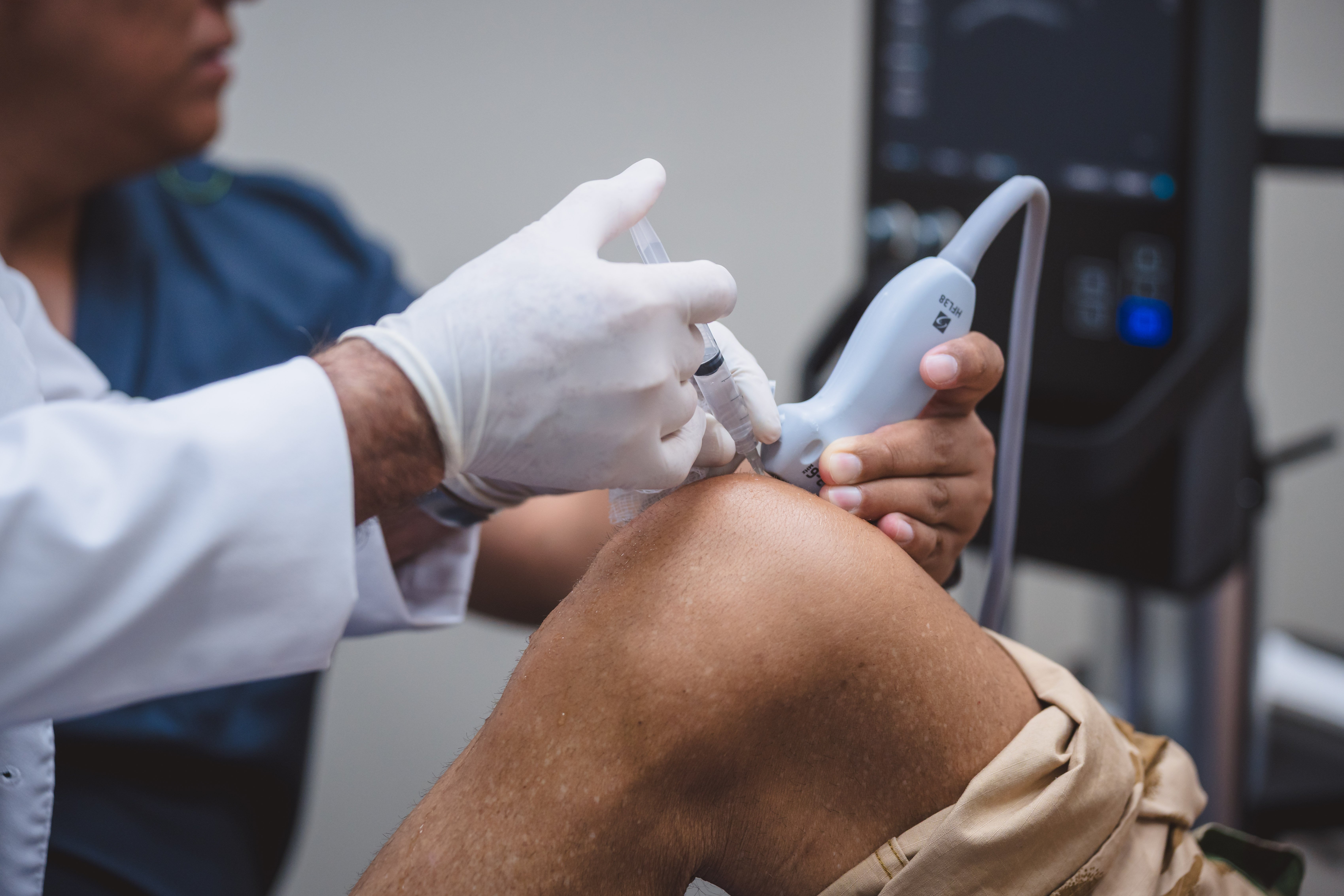

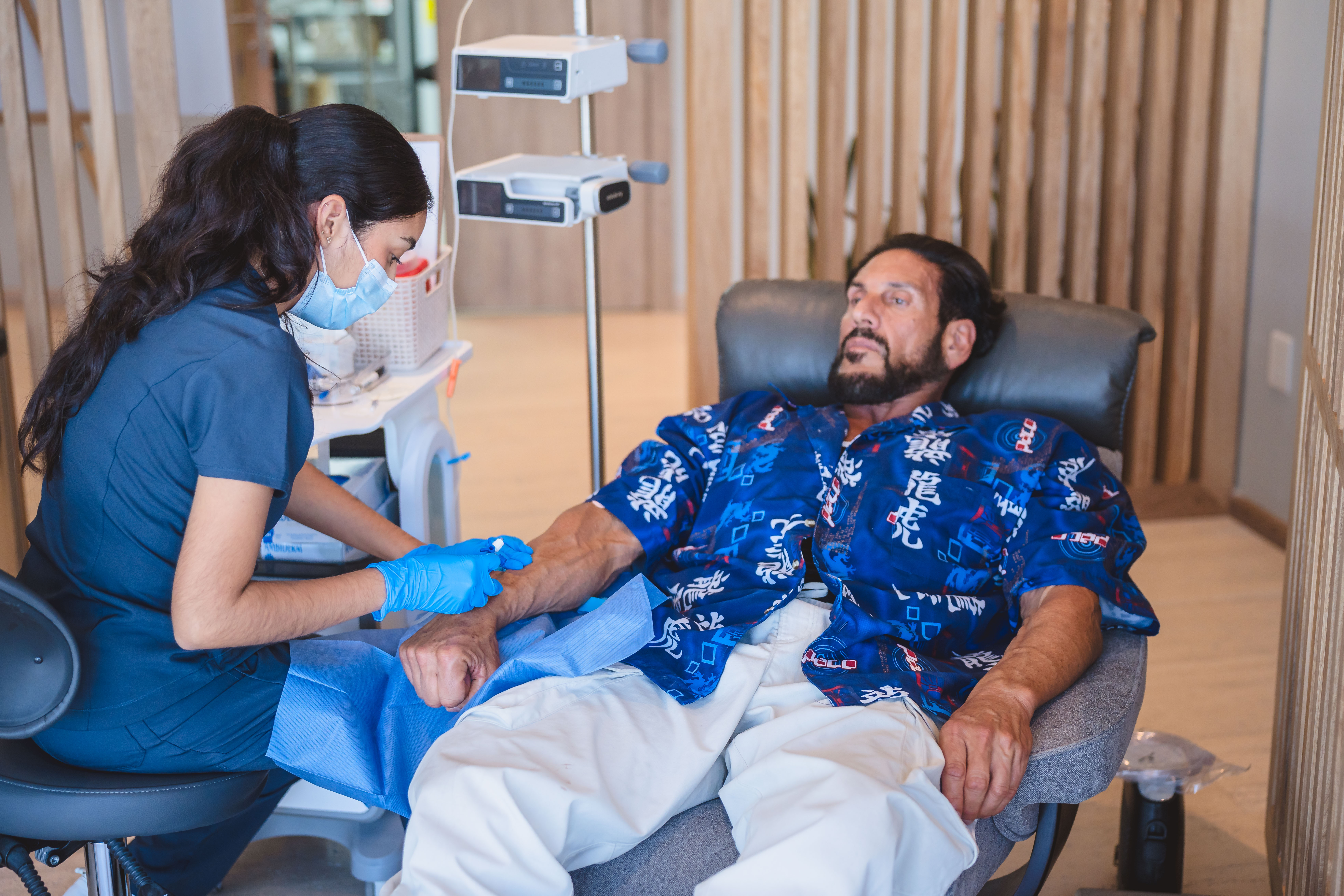

Pillar 1: Federal Licensing and Regulatory Oversight
Regenerative medicine is not a cosmetic service - it is advanced medical care involving living cellular material. That is why Mexico regulates it under COFEPRIS.
What is COFEPRIS?
COFEPRIS stands for the Comisión Federal para la Protección contra Riesgos Sanitarios. It is Mexico’s national health authority responsible for regulating medical procedures, biologics, laboratories, and medical facilities, often described as the Mexican equivalent of the FDA.
In regenerative medicine, COFEPRIS oversight matters because it governs whether a clinic is legally authorized to handle cellular products, operate specialized laboratories, and administer stem cell–based therapies under federally inspected standards.
COFEPRIS does not issue “general approvals” and they are not transferable or sublicensable to another clinic. Each authorization is specific, heavily inspected, and difficult to obtain, especially for a clinic that is not a hospital.
Longevity Medical Institute® COFEPRIS Federal Authorizations
Longevity Medical Institute® currently holds five federal COFEPRIS licenses.
- Medicina Regenerativa (Regenerative Medicine) – Federal authorization to administer stem cell–based regenerative therapies
- Banco de Células Progenitoras o Troncales (Biotechnology Laboratory), through our partnership with ITC Biotechnology, has received federal authorization to process, handle, and scientifically verify stem/progenitor cells, including advanced quality assessment (e.g., flow cytometry)
- Laboratorios Médicos y de Diagnóstico (Clinical Laboratory) – In-house diagnostic blood testing
- Actos Quirúrgicos (Operating Room) – Surgical authorization for sterile operating room procedures
- Resonancia Magnética (MRI) – Licensed diagnostic imaging under federal standards
These approvals are virtually non-existent for a clinic (non-hospital) to obtain. Longevity Medical Institute® is currently the only clinic in all of Mexico with all five federal authorizations in place.
To put this in perspective:
Across all of Mexico in 2025, only two federal regenerative medicine (stem cell administration) licenses were issued - Longevity Medical Institute® and SHA Wellness in Cancún.
In addition, we are the only stem cell clinic in Los Cabos holding both federal authorizations for stem cell administration (Regenerative Medicine) and for a biotechnology stem cell laboratory. This biotechnology laboratory license is even more exclusive, and through our partnership with ITC Biotechnology, we are among only several clinics in Mexico that hold this rare combination for stem cells, uniting regulated treatment with on-site scientific verification.
These approvals represent rigorous inspection, validated equipment and facilities, documented protocols, credentialed staff, and ongoing compliance.
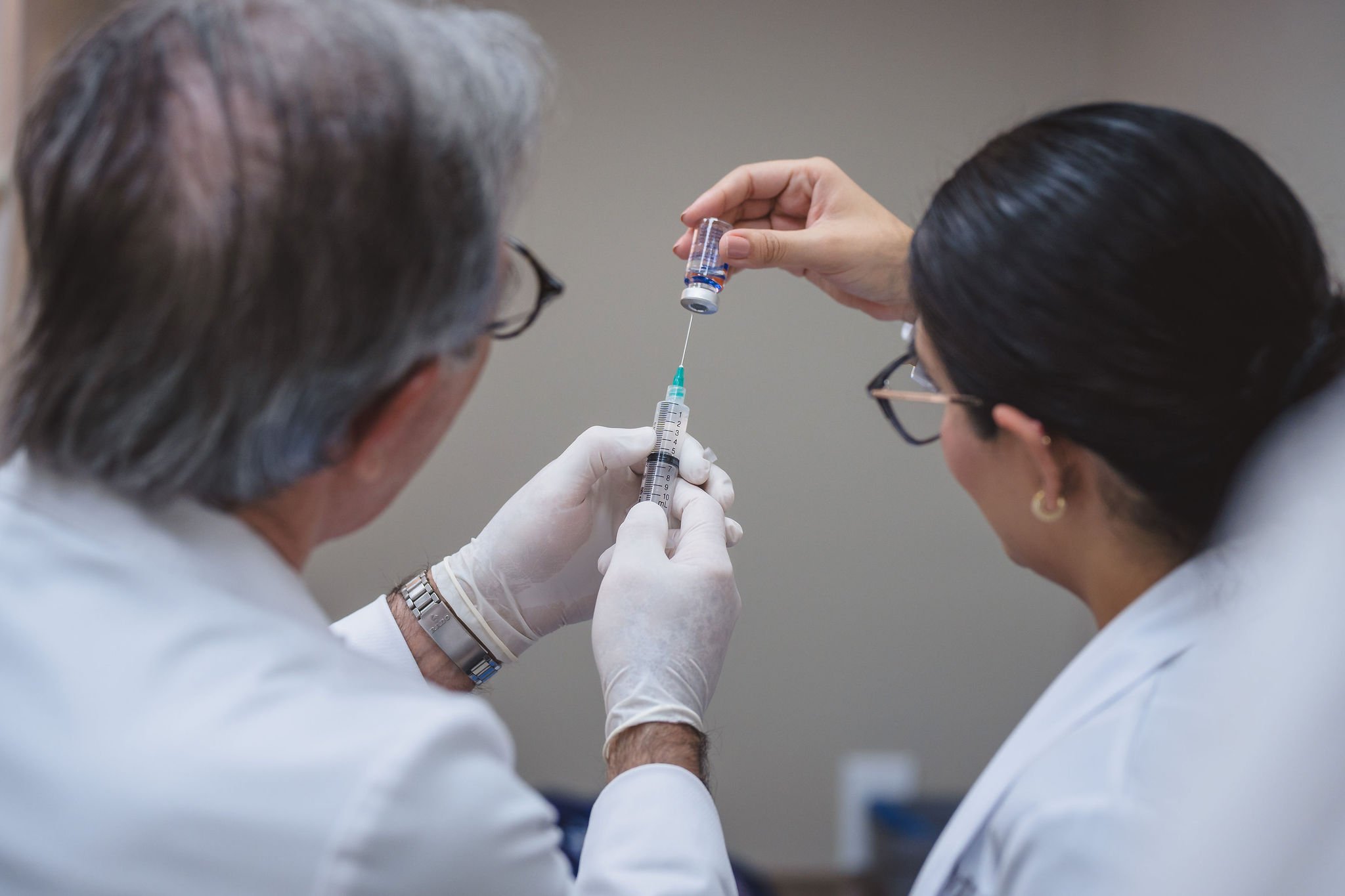



Pillar 2: Transparency at the Cellular Level
One of the most important, and most overlooked, questions in regenerative medicine is simple:
What Exactly is Going Into My Body?
In many clinics, patients are asked to trust broad claims without measurable proof. At Longevity Medical Institute®, transparency is built into the medical process.
An On-Site Biotechnology Laboratory
Our certified biotechnology laboratory (in partnership with ITC Biotechnology) allows for advanced cellular verification before therapies are administered.
Using flow cytometry, we can scientifically evaluate cellular preparations, confirming key cell populations and quality markers with objective measurement.
What Patients Receive: A Flow Cytometry Report
Patients receiving regenerative therapies at LMI can review a flow cytometry report that helps clarify what is present in their cellular preparation.
View Our Flow Cytometer Report
This is how trust becomes tangible:
- Not “trust us” - but here is the data.
- Not vague assurances - but documented verification.
- Not marketing language - but scientific transparency.
Important Safety Note: It was once assumed that administering non-viable stem cells (dead cells) would simply mean “no results,” but research now shows these cells can be recognized by the body as foreign or damaged material and actually trigger an immune or inflammatory response. Instead of being harmless, non-viable cells can activate the immune system’s defense mechanisms, potentially leading to inflammation and unwanted immune reactions - which is why cell viability, safety standards, and transparency are critical in responsible regenerative medicine.
Pillar 3: Physician-Led Regenerative Medicine
Licenses and laboratory verification establish safety, but trust is also placed in the physicians who guide treatment decisions.
At Longevity Medical Institute®, regenerative therapies are not technician-driven or protocol-only. They are physician-led, medically supervised, and grounded in clinical judgment based on the advanced diagnostics we perform.
Dr. Félix Porras, MD – Medical Director
Dr. Félix Porras provides senior oversight for our regenerative medicine program and is an active member of the Mexican Council of Stem Cells for Therapeutic and Research Purposes and the Mexican College of Stem Cells and Regenerative Medicine. He also holds regenerative certifications through ISSCA, IIEMED, and the International Congress for Orthopedic Regenerative Medicine - bringing disciplined, standards-based leadership to stem cell care.
Dr. Fergie Martínez, MD, MSc – Chief of Regenerative Medicine
Dr. Fergie Martínez leads stem cell protocol design and clinical delivery at LMI. In addition to her MD, she holds a rare Master’s Degree in Regenerative Medicine (Universidad Anáhuac) and is certified through ISSCA and the International Congress of Regenerative Medicine, ensuring therapies are guided by specialized training, not trends.
Dr. Luz Flores, MD – Chief of Hematology & Regenerative Transplantation
Dr. Luz Flores brings transplantation-level rigor to cellular therapy. She is a board-certified hematologist with 20+ years of experience in hematopoietic stem cell transplantation and regenerative medicine, with advanced training at Centro Médico Nacional “20 de Noviembre” (ISSSTE) and a diploma in hematopoietic progenitor transplantation from the University of Valencia.
Dr. Janine Zamitiz, MD – Chief of Anti-Aging and Aesthetics
Dr. Janine Zamitiz brings more than a decade of global experience in aesthetic and regenerative medicine, with a clinical focus on Regenerative Aesthetics and how stem cells and exosomes can support skin quality, recovery, and visible aging outcomes. She earned her MD from the Autonomous University of Guadalajara (UAG) and completed dual master’s degrees in aesthetic and anti-aging medicine through TECH (in collaboration with Universitat de Barcelona) and IPPC Zaragoza. She is a member of COMMEL and maintains ongoing regenerative training, including certifications from the International Stem Cell Applications Association (ISSCA).
Together, this leadership team anchors regenerative medicine at LMI in what matters most: medical accountability, verified science, and patient safety.
Why You Should Be Concerned About an Unlicensed Stem Cell Clinic
In Mexico, stem cell therapies are regulated at the federal level by COFEPRIS. If a clinic is administering stem cells without COFEPRIS authorization, it means there is no verified federal oversight confirming they are legally approved, properly inspected, or operating under required handling and safety standards.
What that can mean for patients:
- No federal inspection or accountability
- Unknown sterility and cell-handling standards
- No verified quality controls (what’s actually being administered)
- Higher risk, with fewer protections if something goes wrong
When a clinic or doctor administers stem cell therapy without COFEPRIS authorization, this violates unlicensed medical acts, operating outside an approved scope, and misrepresenting authorized services. And COFEPRIS can respond with loss of the physician's medical license, clinic closure, seizures of biologic materials, and major fines, including criminal investigation if a patient were to be harmed.
You wouldn’t choose an unlicensed clinic in the United States or Canada, so why accept that risk anywhere else? In regenerative medicine, the standard should be the same: licensed, inspected, and accountable.
Trust You Can Verify
In regenerative medicine, trust should never be based on claims alone. It should be built on:
- Federal authorization (COFEPRIS)
- On-site scientific verification (flow cytometry)
- Physician-led decision-making by qualified experts
That’s the standard we uphold at Longevity Medical Institute®, so our patients can move forward with true peace of mind.
OFFER EXTENDED - EXPIRES FEBRUARY 28TH!
OUR MOST COMPREHENSIVE LONGEVITY DIAGNOSTICS
Our most comprehensive Longevity Diagnostics program has become one of our most requested services, with patients planning visits weeks in advance to complete their evaluations. To accommodate this high demand and allow more patients the opportunity to participate, we are extending the offer until the end of February:
- Regular price: $5,750
- Special Price: $3,750
- Save $2,000 (nearly 35%). February only. Limited availability.
Includes:
- Full-Body MRI with DeepRecon® AI
- Advanced Lab Panel measuring 120+ critical biomarkers including cancer, inflammation, hormones, and more
- CADScor® Cardiovascular Assessment
- Mindray AI-Enhanced 12-Lead ECG
- InBody 970 Body Composition Scan
- DermaSensor® Skin Cancer Assessment
- Physician-Led Follow-Up & Personalized Longevity Plan
To schedule your appointment, please contact Ivanna or Ariana, and our team will be happy to assist you with availability and next steps.
Your Feedback Makes a Difference
We're proud to share that we've already received multiple five-star reviews — thank you to everyone who's taken the time to support us!
As a new clinic, your positive feedback not only means the world to us, but it also helps boost our visibility online so more people can discover us.
If you’ve had a positive experience at Longevity Medical Institute, we’d be truly grateful for your review. Select the button below to Leave a Google Review
See more of our articles
Be up to date and check our newest educational materials.

Inside Our State-of-the-Art Surgical Center
Personalized Surgery. Regenerative Healing. One Coordinated Team Centered on You!

Destino Magazine Cover Story: Winter Edition 2026
THE SCIENCE OF LIVING WELL. By Dr. Kirk Sanford, Founder of Longevity Medical Institute.
Start the New Year Right. Save $2,000 on Our Most Comprehensive Longevity Diagnostics!
New Year. New You. This January, experience what may be the most complete longevity diagnostics assessment available today - a comprehensive, physician-led evaluation designed to understand your health from head to toe, long before symptoms dictate the conversation.
Bridging the expertise of traditional medicine with the innovations of tomorrow.
With decades of experience spanning tens of thousands of patient cases, our doctors provide an unparalleled depth of care.